Researchers have been increasingly exploring the promise of three-dimensional (3D) cell culturing, and a recent achievement in Parkinson’s research shows that promise beginning to reach its full potential.
At the Luxembourg Centre for Systems Biomedicine (LCSB) of the University of Luxembourg, researchers have had success growing the type of cells affected by Parkinson’s disease in a 3D environment. Their findings were included in the article “Differentiation of neuroepithelial stem cells into functional dopaminergic neurons in 3D microfluidic cell culture” in the March 30 issue of the journal Lab on a Chip.

Flikr, Kristina D.C. Hoeppner, March 19, 2009
In Parkinson’s, a chronic and progressive movement disorder caused by the malfunction and death of vital nerve cells in the brain, the brain loses dopamine- producing neurons. While these neurons have been cultured successfully in traditional, two-dimensional environments in the past, the 3D environment can offer new insights.
WHEN 3D CULTURING MAKES A DIFFERENCE
As Cindy Neeley, senior staff scientist in field applications at Thermo Fisher Scientific, explains, “The 3D culture mimics the in vivo growth environment more effectively than a 2D culture. For example, 3D cultures can exhibit differentiated cellular functions such as gene and protein expressions that are absent in 2D cultures. Spheroids from 3D cell cultures demonstrate chemical gradients of oxygen, nutrients, and catabolites similarly to those in the microenvironment of in vivo tissue.”
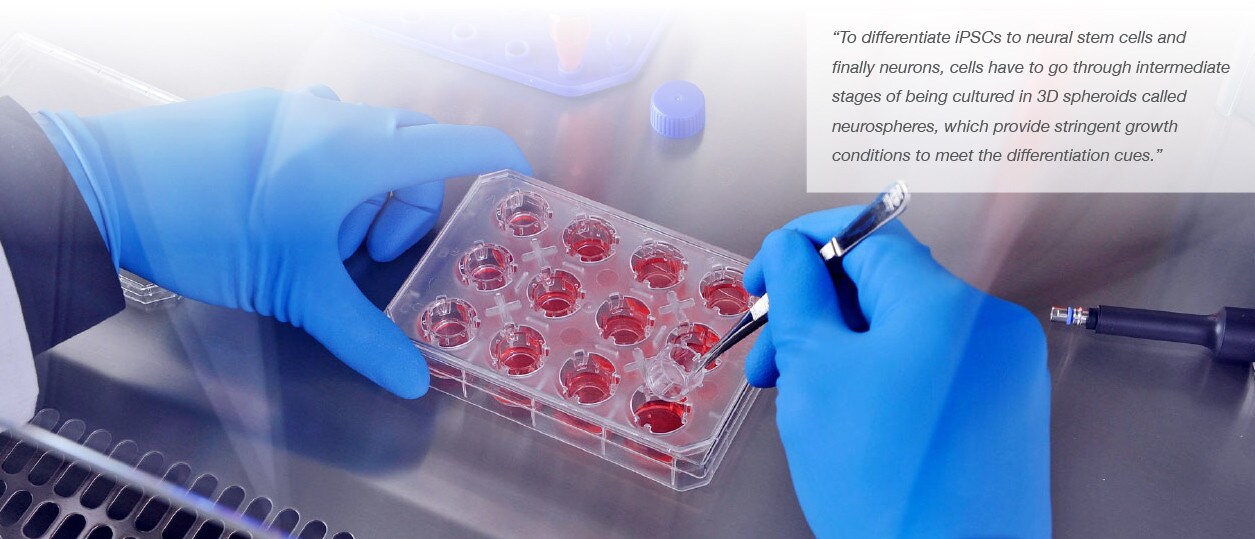
At the University of Luxembourg, the team used a two-step process to generate the desired cells. Regular skin cells were converted to induced pluripotent stem cells (iPSCs). Then, as Professor Jens Schwamborn, head of the LCSB research group Developmental & Cellular Biology, explains, “By adding suitable growth factors, the iPSCs can then be converted in a second step into neural stem cells. These are the starting cells we use in the microfluidic culture.”
Neeley adds, “To differentiate iPSCs to neural stem cells and finally neurons, cells have to go through intermediate stages of being cultured in 3D spheroids called neurospheres, which provide stringent growth conditions to meet the differentiation cues.”
After 30 days of growth, 91 percent of the cells in the culture are neurons, with about 20 percent having become the desired dopaminergic neurons. Morphological and immunological tests have confirmed the findings.
Choosing THE RIGHT SURFACE
In cancer research, 3D culturing has already taken hold as a critical tool for effective, productive research. Working in three dimensions is key to encouraging the formation of spheroids, which best replicate the natural formation, growth, and behavior of cancer tumors.
From a Thermo Fisher Scientific Accelerating Science post:
“The two-dimensional monolayer cell culture is well known and it results in a comforting uniformity—cells exposed to consistent concentrations of oxygen and nutrients, relatively uniform growth rate, and equal exposure to extracellular signals. None of this, however, replicates the in vivo growth environment of tumors, whose surfaces and hypoxic cores demonstrate distinct growth patterns.”
Thermo Fisher Scientific has provided support to other researchers working in the field of Parkinson’s research. A Thermo Fisher Scientific research team partnered with the Parkinson’s Institute in Sunnyvale, California, to develop Parkinson’s disease model systems using donor fibroblasts that have been collected at the institute.
In the joint effort, staff scientist Chetana Revankar, PhD, used embryoid body (EB) cultures to generate neural stem cells. The work was covered in early 2015 in R&D Magazine.
GETTING STARTED WITH 3D CULTURING
The most important aspect to successful 3D culturing is using an ultra-low-binding surface coated with a neutrally charged hydrophilic polymer. Thermo Fisher Scientific offers a Surface Selection Guide for researchers exploring the best environments in which to culture cells.
For 3D culturing, there are several options for researchers to consider. The Thermo Fisher Sphera line includes ultra low-binding flasks, dishes, multi-dishes, and plates. Other 3D culture systems include Thermo Fisher Scientific’s Nunc Cell Culture Insert, a porous membrane-based culture platform that can be used to form 3D skin tissue in multi-well dishes.
The Thermo Scientific UpCell Surface allows researchers to generate tissues by growing and stacking cell sheets. It has been used to generate corneal tissues for treatment of eye diseases and to create myocardial tissues to restore damaged heart tissue.
Additionally, hydrogel-based 3D scaffold materials such as the Geltrex and AlgiMatrix that mimics physiological characteristics of the extracellular matrix (ECM) in vivo.
THE “RIGHT DIRECTION” FOR PARKINSON’S WORK
So, what does the Luxembourg achievement mean to Parkinson’s research? “This is the initial step towards the right direction,” Neeley notes. But “lots of work still needs to be done to ensure proper differentiation of iPSCs to the right types of neurons, as well as to validate the characteristics and functionalities of the regenerated neurons, their in vivo efficacy and safety, and more.”
What’s next for the team? Because cells from individual Parkinson’s patients can be used, Fleming notes that the finding is “an important step towards personalised drug development.” The team plans to study cells from patients and begin testing active pharmaceutical ingredients before progressing to testing promising substances in mice.
Want to learn more about 3D cell cultures? Explore our on-demand webinar: Harnessing new dimensions in your research: Growing 3D cultures consistently and repeatedly
Leave a Reply